Blood Culture Collection FAQs
- Who should receive a blood culture test?
- Why is a blood culture needed?
- How accurate is a blood culture test?
- Why are two specimens required from two separate sites?
- Can skin antisepsis prevent blood culture contamination?
- How can I prevent microbes from within the skin from entering the blood culture bottle?
- How do I maintain skin antisepsis while drawing a blood culture?
- How will I know if I am properly in the vein for optimal blood culture collection?
- How much blood must I collect for an adult blood culture?
- How much blood must I collect for a Pediatrics/Neonatal blood culture?
- Do I need to disinfect the culture bottles?
- Which blood culture bottle do I use first?
- What happens if I don’t get enough blood or if I overfill a bottle?
- How can I be sure to draw the correct amount?
- How many blood culture tests are needed per patient?
- How is a Laboratory confirmed bloodstream infection diagnosed?
- What is the best method for blood culture collection?
- If there are other lab specimens ordered for the patient, is there a preferred order to the collections?
- Do I shake the collected specimen in the bottles?
- How should a blood culture specimen be labeled?
- How do contaminated blood cultures impact false positive CLABSI reporting?
Who should receive a blood culture test?
Blood cultures are commonly collected when patients have fever, chills, leukocytosis, septic shock, suspected endocarditis or prior to starting antimicrobial treatment in elderly or immunocompromised patients. In U.S. hospitals, blood culture tests are used upon arrival to rule out a community acquired bloodstream infection (BSI), as a BSI that occurs during hospitalization is considered a preventable adverse event, which could threaten reimbursement for the patient’s care.
Why is a blood culture needed?
A blood culture is used to help determine if a patient has a bloodstream infection, also known as bacteremia or septicemia. This condition can be life-threatening. Blood is naturally a sterile fluid. Bacteria should not be present in the bloodstream. Ideally, a blood culture test yields an aetiological diagnosis and provide the opportunity to perform antimicrobial susceptibility testing to guide proper antimicrobial therapy, an important aspect of antibiotic stewardship.
How accurate is a blood culture test?
Unfortunately, blood culture contamination is a persistent problem in gathering reliable test results. Approximately 1/3 of positive blood culture tests in the US are actually false-positives triggered by contamination.
Why are two specimens required from two separate sites?
To mitigate this perceived inevitability, 2 cultures are drawn for each collection from two separate insertion sites. This helps to distinguish true bloodstream infection (in which both specimens will be positive with the same organism) vs. positive results from contamination (in which only one specimen will be positive.)
Can skin antisepsis prevent blood culture contamination?
Many bacteria colonize the skin as part of the body’s natural defense against illness. Proper aseptic technique can be used to decontaminate the patient’s skin surface before collecting the blood culture. If disinfecting steps are not properly followed, skin bacteria can transfer to the bottle and cause false positive results. However, even if skin antisepsis is followed, microbes within the dermis layer that cannot be reached by surface disinfection methods, may be transferred in the specimen with the skin plug cored out by the butterfly needle.
How can I prevent microbes from within the skin from entering the blood culture bottle?
Research indicates that using a method, sometimes referred to as “a waste,” to prevent the initial flash of blood, as little as 0.15ml, from entering the bottle will greatly reduce contaminated culture rates. This can be accomplished with a blood culture collection set with integrated passive flash technology that sidelines the initial bit of blood.
How do I maintain skin antisepsis while drawing a blood culture?
Properly disinfect the venipuncture site per hospital protocol. Maintain a “hands-off” approach after the prep procedure and do not palpate the site after antisepsis. If it is necessary to touch the cleansed site, a sterile glove should be applied following sterile procedure.
How will I know if I am properly in the vein for optimal blood culture collection?
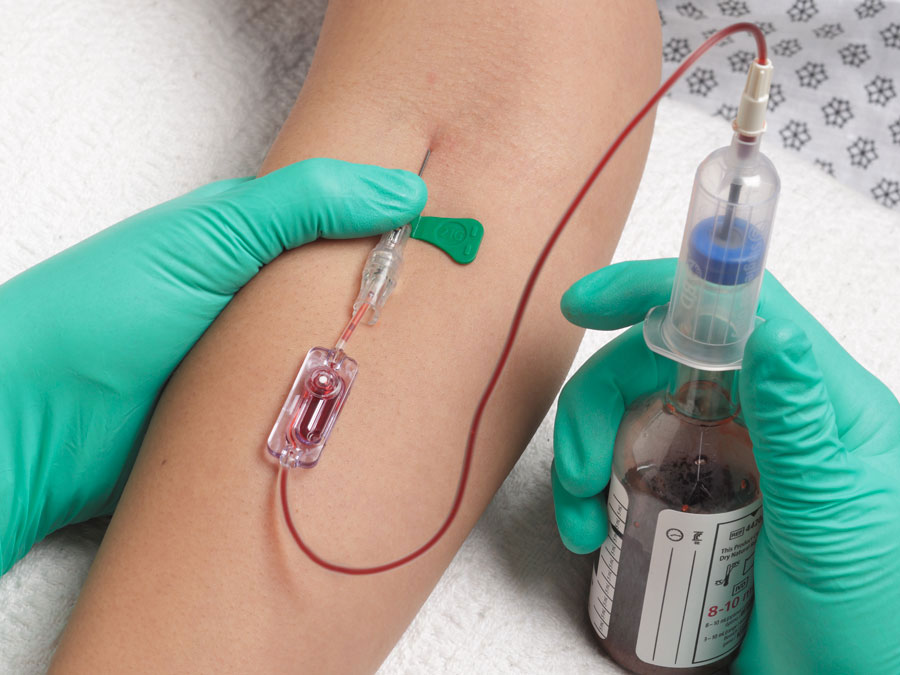
For a peripheral draw, use a butterfly collection set to perform the venipuncture. Watch for the blood to “flash” back into the tubing to confirm that you are in the vein.
How much blood must I collect for an adult blood culture?
A blood culture set is defined as two bottles, an aerobic bottle and an anaerobic bottle. Two blood culture sets (a total of 4 bottles) should be drawn. For adults, collect 8-10 ml of blood per bottle.
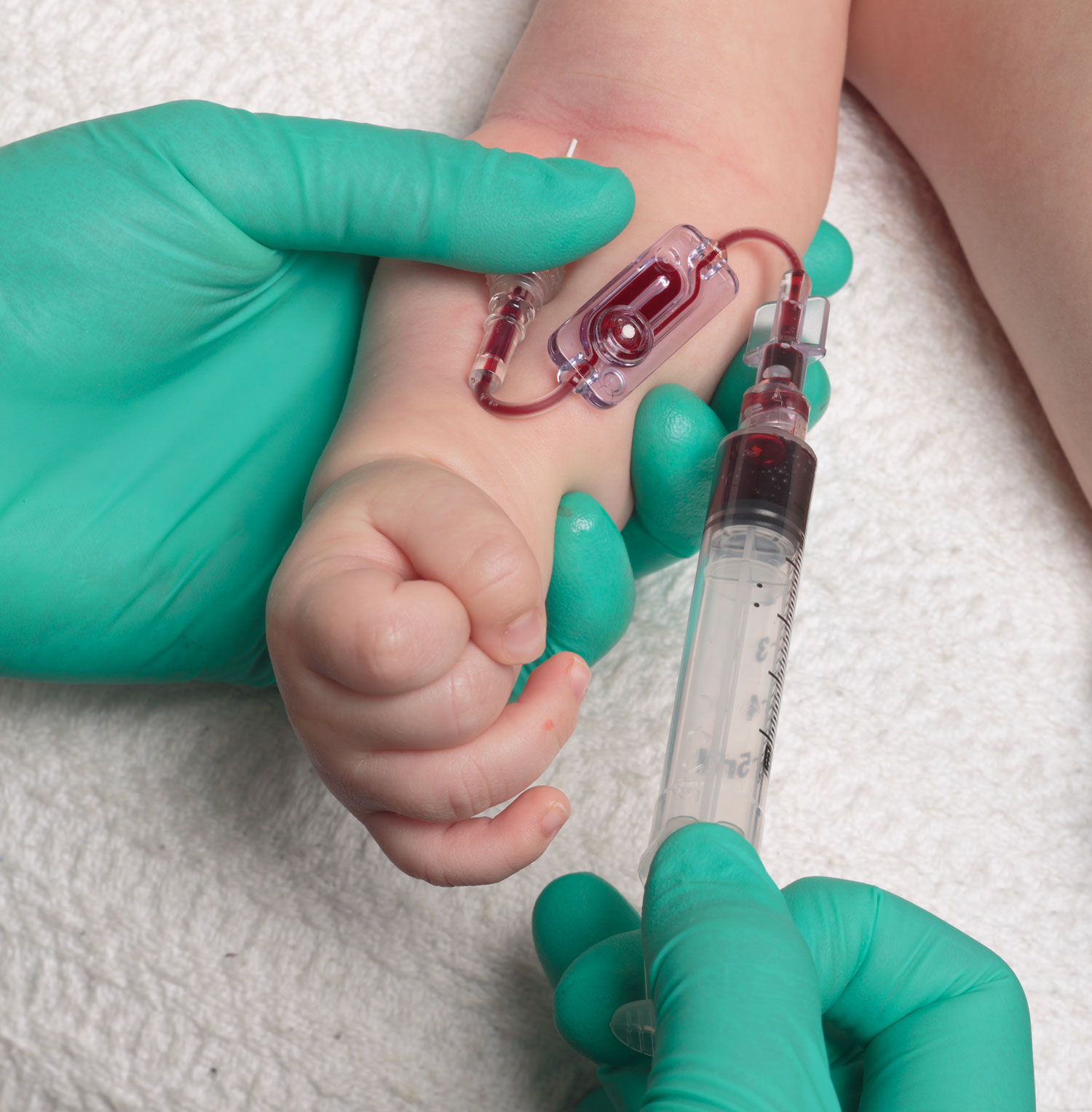
How much blood must I collect for a Pediatrics/Neonatal blood culture?
Facilities follow different policies for pediatrics and neonatal blood collection. One policy is to go by the patient’s age under 12 months. A one-month-old baby will require 1ml, a two-month-old baby will require 2ml and so on. Other facilities follow a weight-based policy. For example, for a patient <= 9kg, use the Aerobic Bottle to collect 1 ml minimum to 4 ml maximum and for patients >=9.1 kg use the Anaerobic Bottle to collect 4 ml minimum to 10 ml maximum.
Do I need to disinfect the culture bottles?
Yes. Blood culture bottles are not considered sterile, even with the dust cap in place. Remove the dust covers from the bottles and disinfect bottle tops per hospital policy and allow them to dry.
Which blood culture bottle do I use first?
The aerobic bottle should be inoculated first as there is about 0.5 cc of air in the line of the collection set and sometimes it is difficult to obtain 8-10 cc of blood per bottle (16-20 cc/set). The aerobic bottle is the more critical one to inoculate short samples into.
What happens if I don’t get enough blood or if I overfill a bottle?
It is important not to underfill or overfill the bottles as this can adversely affect the results.
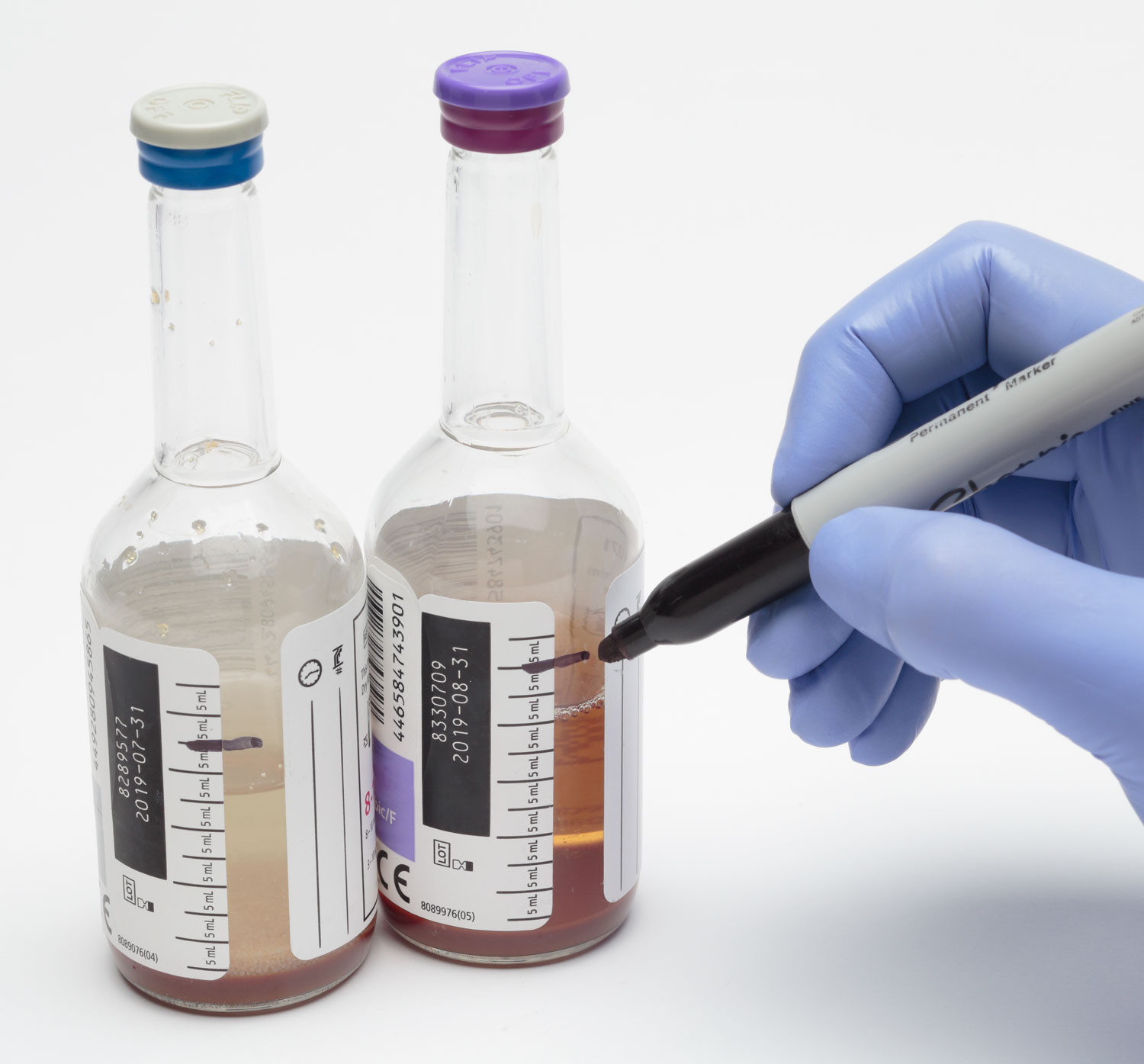
How can I be sure to draw the correct amount?
Blood culture bottles must be standing upright to avoid back flow of the broth medium and ensure that an appropriate volume of blood is added. Use the tick marks on the edge of the label that indicate approximately 5 cc increments. Some blood culture bottles have a “fill line” to help identify your target volume. If the blood culture bottles do not have a fill line, be sure to pre-mark the bottles to two tick marks above the broth to act as your fill line target.
How many blood culture tests are needed per patient?
For adults with suspected bacteremia, draw two sets of blood culture bottles (aerobic and anaerobic) from two separate venipuncture sites. Maximum of 4 cultures. Two or more sets of blood cultures are defined as a blood culture series.
How is a Laboratory confirmed bloodstream infection diagnosed?
There is 1 positive blood culture with recognized pathogen from a venipuncture or more than 2 blood cultures drawn on separate occasions return positive for the same organism plus clinical symptoms.
What is the best method for blood culture collection?
Whenever possible, a peripheral sample using venipuncture is preferred. Blood specimens obtained from existing intravascular lines are to be avoided as they yield increased rates of contamination. When necessary, a sample can be drawn from a freshly placed peripheral IV although the potential for contamination is increased.
If there are other lab specimens ordered for the patient, is there a preferred order to the collections?
Yes. If other blood is required for additional laboratory tests, draw the additional tubes after the blood cultures are obtained. Drawing additional labs prior to collecting blood cultures can result in a contaminated blood culture.
Do I shake the collected specimen in the bottles?
No. Do not shake them. After the specimens are collected in the blood culture bottles, invert the bottles slowly several times to gently mix the sample thoroughly.
How should a blood culture specimen be labeled?
Each hospital protocol may differ slightly but in general, you will label the bottle(s) with patient name, patient ID, time, date, and location of site of specimen collection, such as “right arm” or “left arm.” For line draws, always indicate which line and/or port. Place the label vertically on the bottle and DO NOT place label over the barcode on the blood culture bottle.
How do contaminated blood cultures impact false positive CLABSI reporting?
All acute care hospitals must report CLABSIs to the CDC National Healthcare Safety Network (NHSN). Contaminated blood cultures pose a serious problem to accurate reporting. If a patient has a central line, a contaminated blood culture can result in the reporting of a CLABSI. With over 1.2 million contaminated blood cultures occurring in the U.S. annually, the frequency and associated cost of unnecessary CLABSI reporting is significant. (https://www.aha.org/education-events/impact-and-
ML-097 Rev A