Passive Engineering Controls Result in Sustained 66% Reduction in Blood Culture Contamination
Monica Baxter, Carolyn Cook and Angie James from St. Mary’s Regional Medical Center, Russellville, AR.
Background
Blood culture testing is an important diagnostic tool in identifying the presence of microbes in the bloodstream. Tests are frequently contaminated, leading to false-positive results. Blood culture contamination can result in unnecessary antibiotic treatment, extended hospital length of stay, and patient exposure to hospital-acquired conditions.
Methods
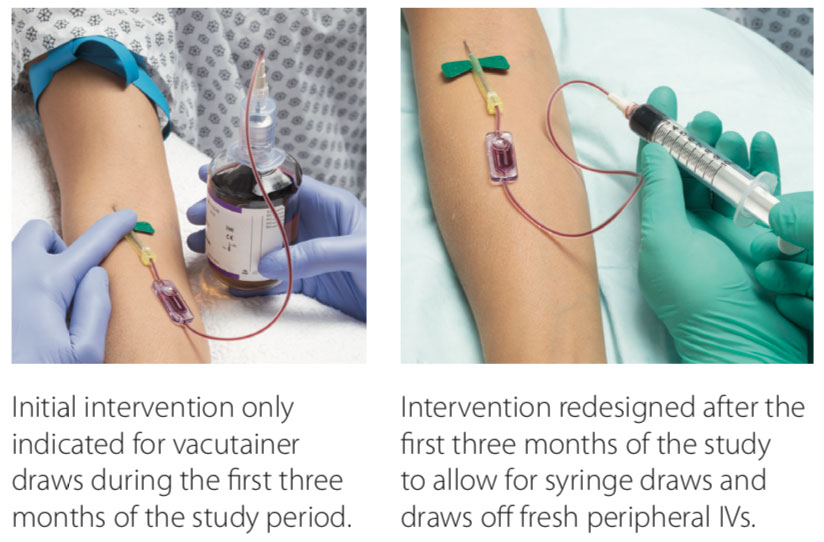
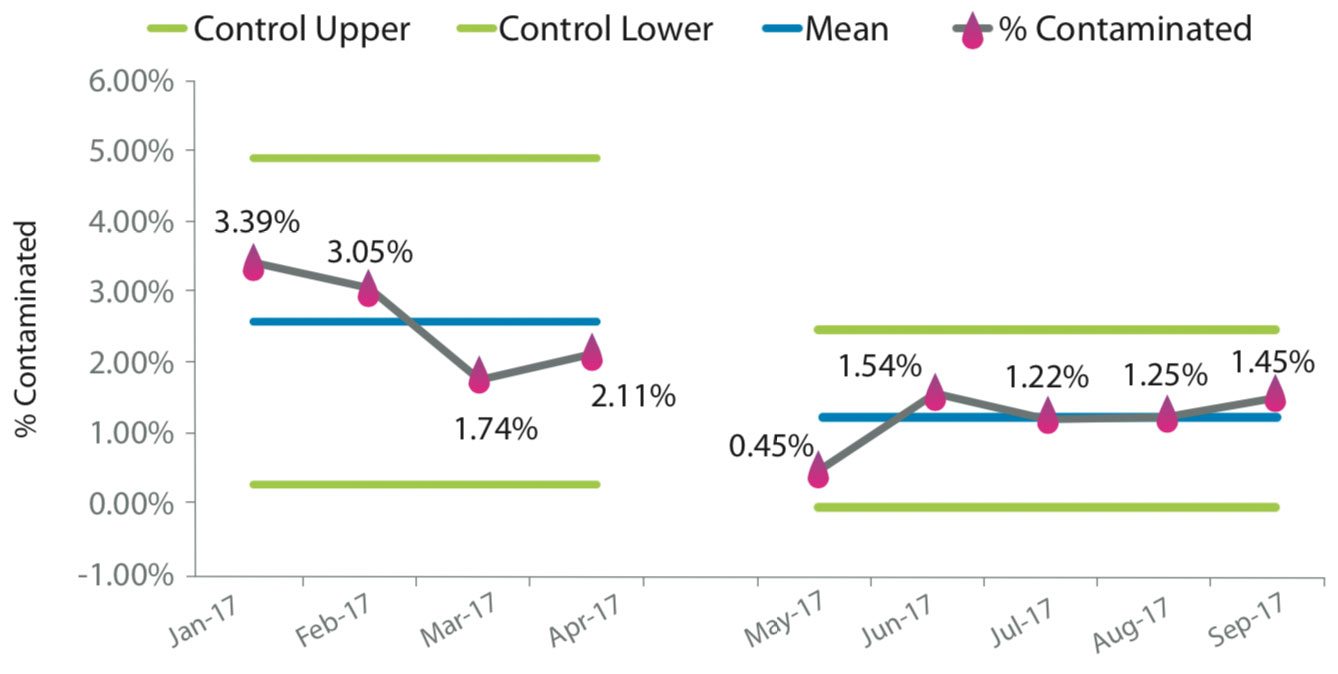
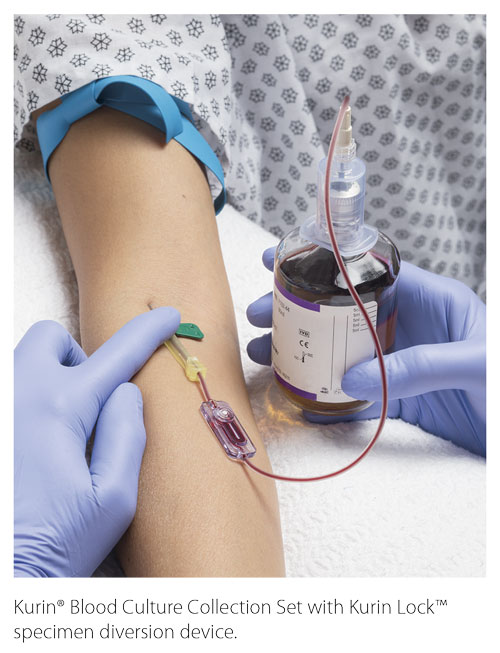
St. Mary’s Regional Medical Center (SMRMC) in Russellville, Arkansas, struggled with blood-culture contamination rates, with an average of 6.8% from 2014 to 2018. Ongoing staff education yielded a reduction to an average of 5%. In an effort to reduce the contamination rates, our facility elected to try a novel specimen diversion device. Laboratory and emergency department (ED) staff were educated on the diversion device prior to the initiation of the trial period. Compliance with the diversion device averaged 70%–75% during the trial period. Monitoring of contaminations was added to our daily safety huddle to provide a quick turnaround time for false-positive education to specific clinical staff.
Results
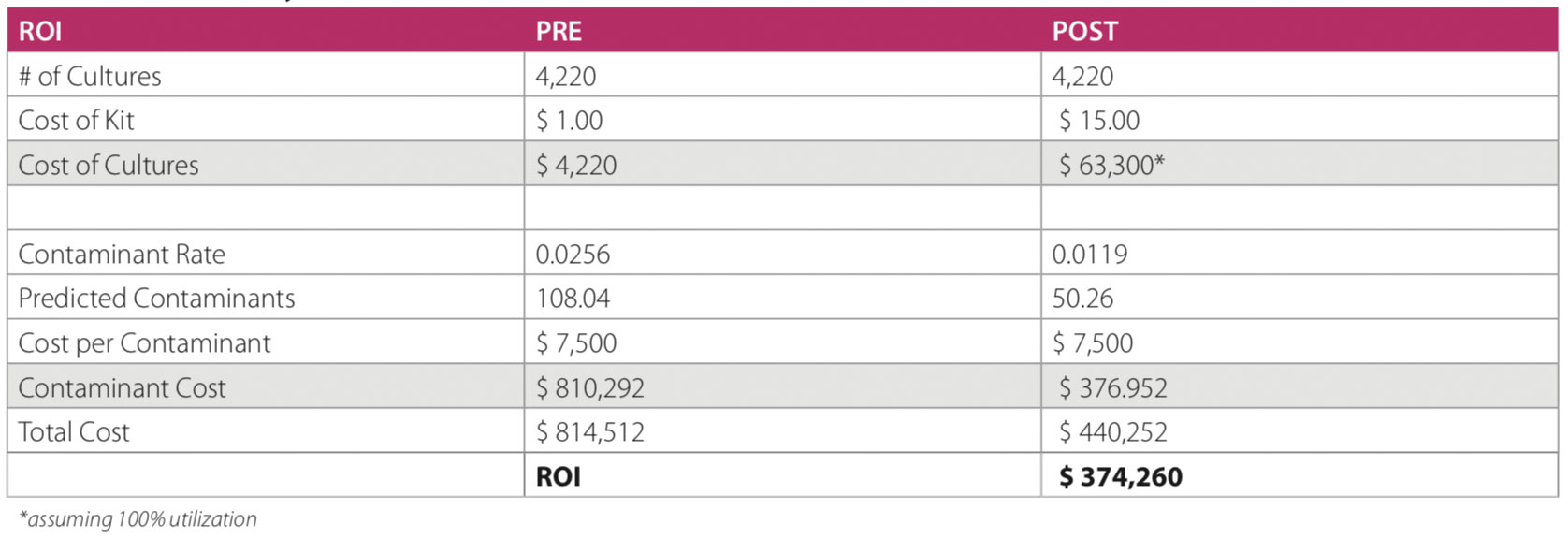
The results were significant, with a decrease in contamination rates from 4.93% to 1.66%—a 66% reduction. Improved blood culture testing has several advantages: best practice for patient care is first and foremost, along with other financial benefits for the facility. Several articles have estimated the cost of a contaminated culture to be $3,000–$10,000 per event; SMRMC has adopting an estimated cost of $4,000. The number of cultures at our hospital averages ~4,400 per year, and these results suggest a savings of >$500,000 per year (as contaminations on an annual basis fell from 217 to 73). With this intervention, 144 patients were spared from receiving unnecessary antibiotics as a result of a false-positive blood culture testing.
Conclusions
We conducted a brief analysis to determine whether there was any obvious change in length of stay for patients with a false-positive blood culture compared to those with true negative results. In analyzing data for 3 different months, patients with contaminated cultures spent an average of 3.97 additional days in the facility. In conclusion, the implementation of this specimen diversion device significantly lowered our contamination rates, was integrated into practice, and has provided clinical and financial benefits.

 Michael Allain, MS, RN, ACNS-BC, CCRN, Crouse Hospital
Michael Allain, MS, RN, ACNS-BC, CCRN, Crouse Hospital